Design, synthesis and structure-activity relationship of a series of novel BCR-ABL inhibitors
-
摘要:
通过对现有的BCR-ABL变构抑制剂阿西米尼(asciminib)进行结构优化研究,得到N-苯基吲哚啉-5-甲酰胺分子骨架Ⅰ,并以该分子骨架为基础,利用分子对接辅助设计合成化合物1~12,使用ESI-MS和NMR对其进行结构表征,后续采用CCK-8法测定目标化合物在体外抗BCR-ABL1依赖型Luc-Ba/F3细胞增殖能力。最终筛选出高活性先导化合物1,针对该化合物在后续成药性评价中暴露出的清除率高、半衰期短等问题进行了其成药性质优化,引入亲水性基团,后续设计并合成化合物13~22,其中化合物17具有较好的细胞抑制活性,清除率较低,半衰期较长,有望作为临床候选化合物展开进一步的生物活性和成药性的评价。
-
关键词:
- 慢性髓细胞性白血病 /
- BCR-ABL融合蛋白 /
- 酪氨酸激酶抑制剂
Abstract:In this study, molecular skeleton I N-phenylindoline-5-formamide was obtained by optimizing the structure of the existing allosteric BCR-ABL inhibitor asciminib. Based on this molecular skeleton, compounds 1-12 were designed and synthesized assisted by molecular docking. After characterizing their structures using ESI-MS and NMR, the anti-BCR-ABL1-dependent Luc-Ba/F3 cell proliferation activity of the target compounds in vitro was determined by CCK-8 assay. Finally, highly active lead compound 1 was screened out. For high clearance rate and short half-life period exposed in subsequent druggability evaluation, its druggability was optimized by introducing hydrophilic groups. Afterwards, compounds 13-22 were designed and synthesized. Compound 17 presented high cell inhibitory activity, low clearance rate and long half-life, and is expected to be used as a clinical candidate for further evaluation of biological activity and druggability.
-
慢性髓细胞性白血病(CML)是白血病4种亚型中的一种,绝大部分的CML的发生与发展都与BCR-ABL融合蛋白有关[1−2]。BCR-ABL融合蛋白是由9号染色体和22号染色体易位形成的费城染色体所表达产生的[3],它相较于c-ABL激酶具有更强的持续性的酪氨酸激酶活性,能够使自身和相关信号通路的信号蛋白磷酸化,从而激活相关信号通路,最终导致了CML的发生[4−5],因此BCR-ABL融合蛋白是治疗CML的重要靶点。
酪氨酸激酶抑制剂(TKIs)通过靶向结合BCR-ABL融合蛋白,来抑制其激酶活性,用于治疗CML。TKIs通常可分为ATP竞争性抑制剂和变构抑制剂两大类:ATP竞争性抑制剂靶向结合BCR-ABL融合蛋白的ATP结合位点,通过抑制激酶的自磷酸化来控制白血病的发展;BCR-ABL变构抑制剂则是通过与肉豆蔻酰口袋结合,使BCR-ABL融合蛋白处于失活的构象,进而起到对激酶的抑制作用[6]。随着ATP竞争型抑制剂的使用与发展,相应的耐药性突变也在不断出现,例如T315I突变,使得大部分ATP竞争型抑制剂都无法有效治疗该类型突变的患者,而 BCR-ABL变构抑制剂的研发为克服耐药性突变提供了新的方向。
阿西米尼(asciminib)是第1个上市的BCR-ABL变构抑制剂[6−7],它通过和肉豆蔻酰口袋结合,使BCR-ABL1处于非活性构象,从而起到抑制作用。一项临床试验表明,阿西米尼对于CML-CP患者具有相当好的疗效,92%的CML-CP患者得到完全血液学缓解,54%的患者得到完全细胞学缓解,45%的患者得到完全分子学缓解,同时阿西米尼可能用于替代治疗那些对普纳替尼产生耐药性或发生不可接受不良反应的患者[8],阿西米尼对包括T315I在内的绝大部分ATP结合位点的耐药性突变有很强的抑制活性,但是与第3代ATP竞争性抑制剂普纳替尼(ponatinib, Luc-Ba/F3 BCR-ABL1WTGI50=0.37 nmol/L;Luc-Ba/F3 BCR-ABL1T315IGI50=2.0 nmol/L)和奥瑞巴替尼(olverembatinib, Luc-Ba/F3 BCR-ABL1WT GI50=0.34 nmol/L;Luc-Ba/F3 BCR-ABL1T315I GI50=0.68 nmol/L)[9]相比,阿西米尼对两种融合细胞的GI50分别仅为1.0 和25 nmol/L[6]。根据美国食品药品监督管理局(FDA)数据以及相关文献显示,阿西米尼的推荐使用剂量为每日80 mg,对于T315I突变患者推荐使用剂量为每日200 mg[10],而奥瑞巴替尼的推荐使用剂量仅为每2日40 mg[11]。本研究旨在通过对阿西米尼结构改造以获得对两种融合细胞具有更高抑制活性的化合物,降低药物使用剂量,提高患者生活水平,同时扩大BCR-ABL变构抑制剂的结构多样性。
1. 实验部分
1.1 试剂与仪器
1200/6110 液质联用仪(美国安捷伦公司); 400 MHzⅢ 核磁共振仪(德国布鲁克公司); ISO-ISV 过柱机(瑞典Biotage公司);SpectraMax Plus 384全波长酶标仪(美国MD公司)。
4-氯二氟甲氧基苯胺,5-吲哚甲酸甲酯(上海毕得医药科技股份有限公司);5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-1-H-吡唑,1,2,3,4-四氢喹啉-6-甲酸甲酯(江苏艾康生物医药研发有限公司);肝微粒体(德国默克生命科学公司); CCK-8试剂盒(北京兰杰柯科技有限公司);快速液相制备色谱柱(常州三泰公司);氘代试剂由核磁检测方提供,其他试剂均为市售分析纯。
1.2 实验思路
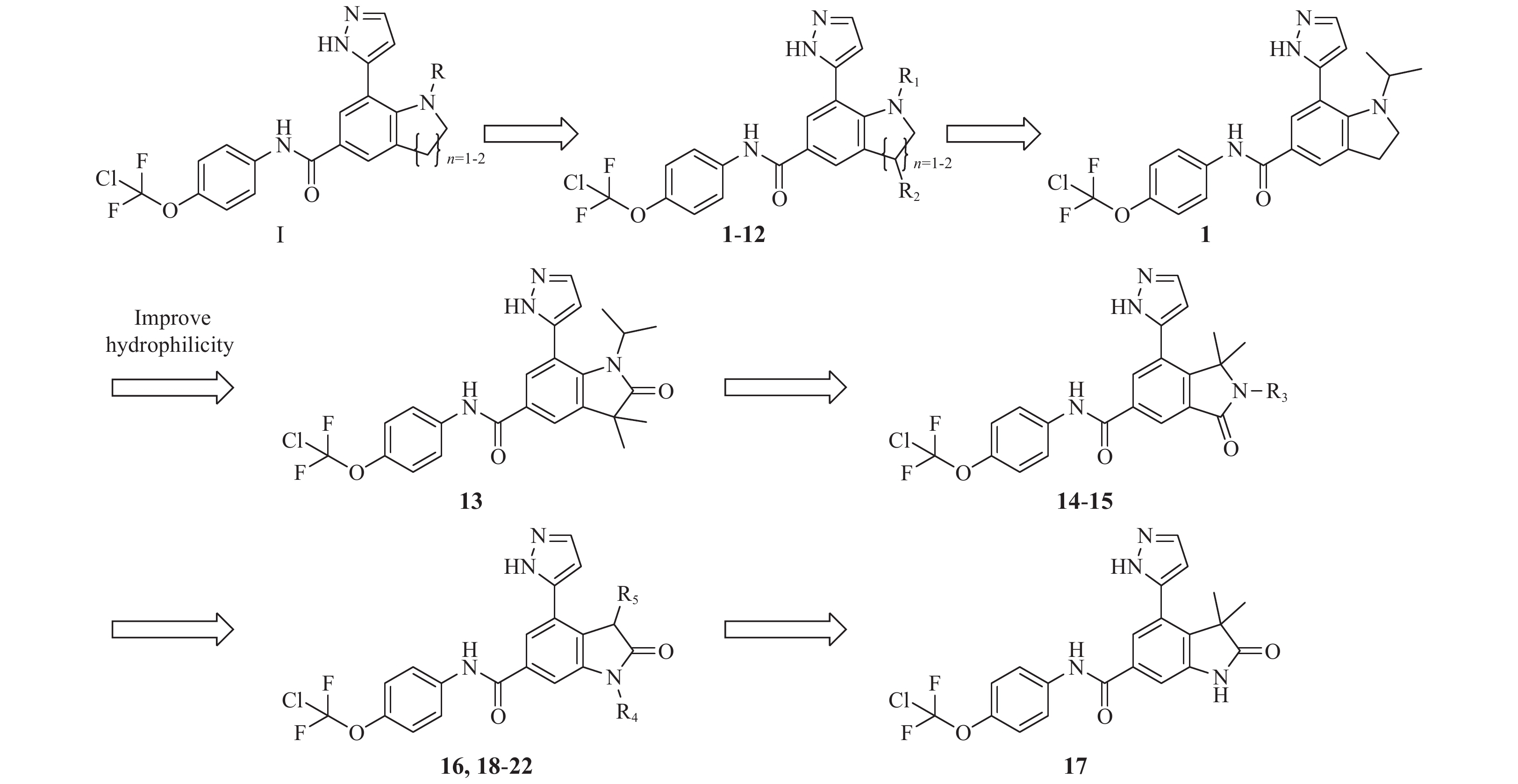
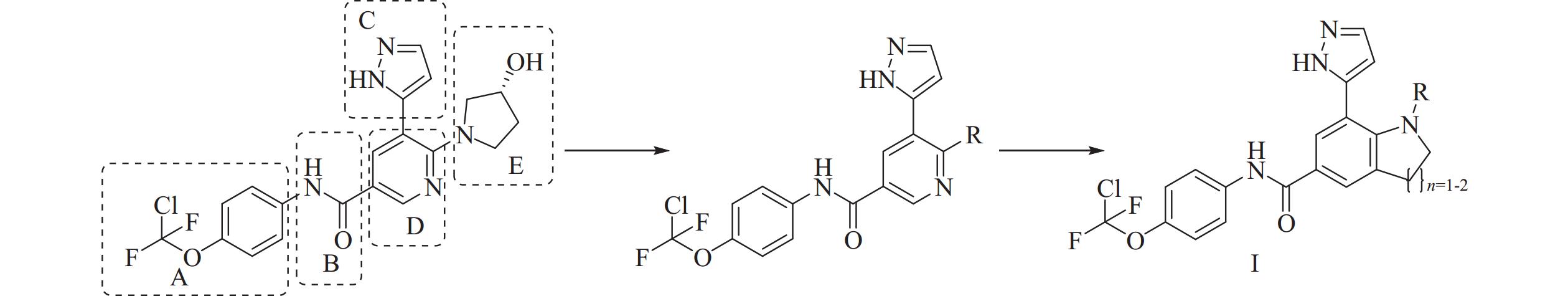
本研究在前期研究中已经对化合物阿西米尼进行初步的结构改造,结果表明A、B、C 3个部分经过官能团的替换会导致化合物对两种融合细胞的抑制活性下降。所以在保留A,B,C 3个部分的前提下将D和E合并为新的并环母核,获得化合物分子骨架Ⅰ(图1)。
在分子骨架Ⅰ的基础上借助分子对接的方法辅助设计出化合物1~12,通过体外活性实验筛选出的高活性化合物1,并对化合物1进行进一步的拓展设计出化合物13~22 (图2)。
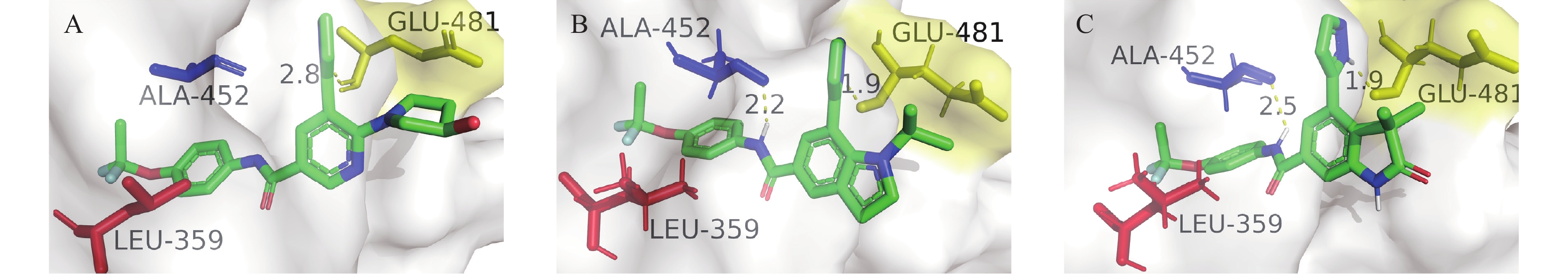
通过Pymol软件将化合物1和化合物17的结合特征可视化,结果表明,化合物1的CF2ClO-基团伸入口袋内部与亮氨酸L359残基通过极性连接,酰胺结构中的-NH-与丙氨酸A452残基形成氢键,吡唑环与谷氨酸E481残基形成氢键(图3-B),而母核N上的取代基起到的主要作用是提供一个合适的位阻使吡唑环发生偏转,化合物17与口袋结合方式与化合物1相似(图3-C)。这与阿西米尼与靶点结合方式相似(图3-A),吡唑环与谷氨酸E481形成氢键。模拟结果表明目标化合物与BCR-ABL靶蛋白有较强的亲和力。
1.3 化合物1~22的合成
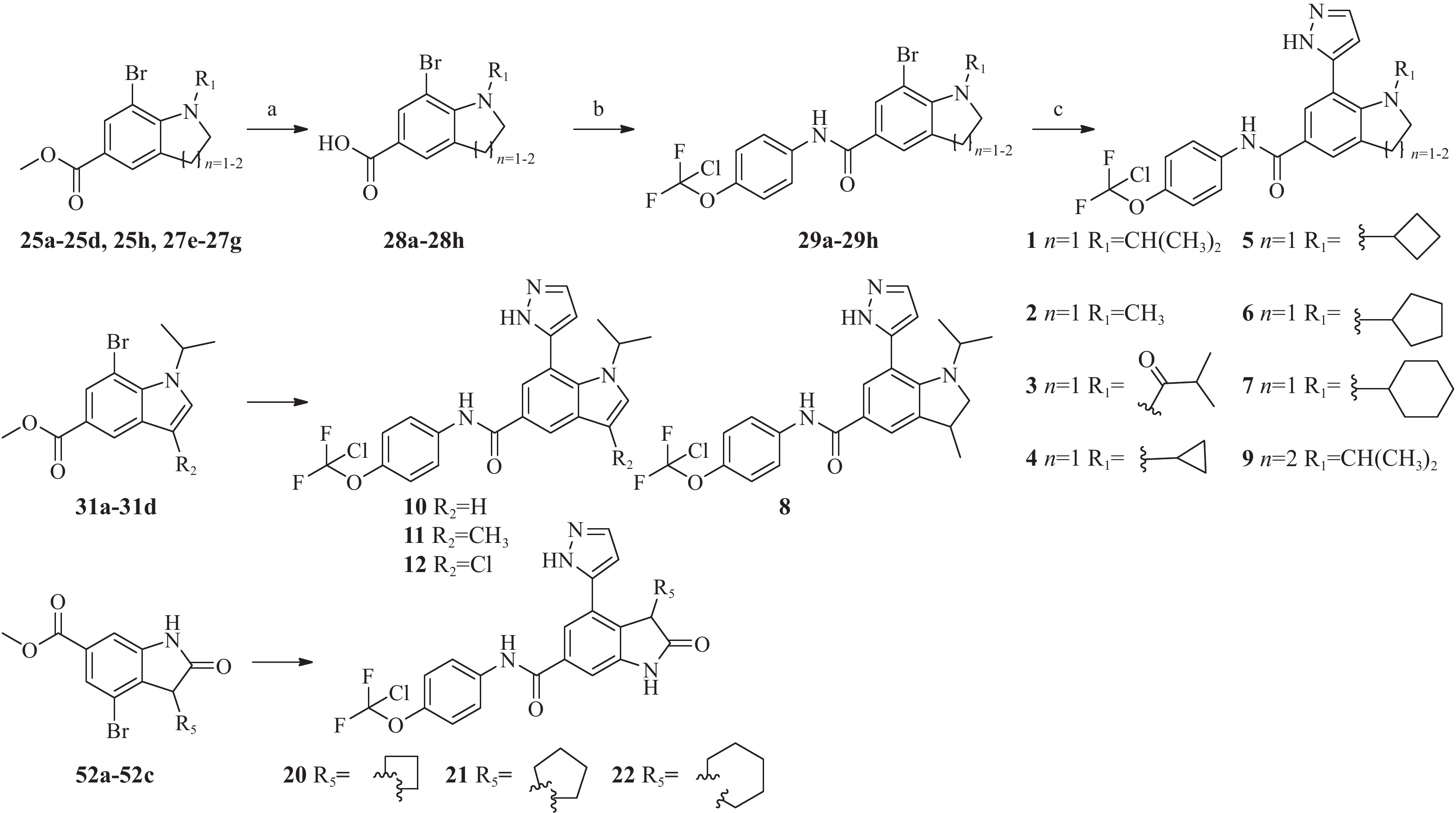
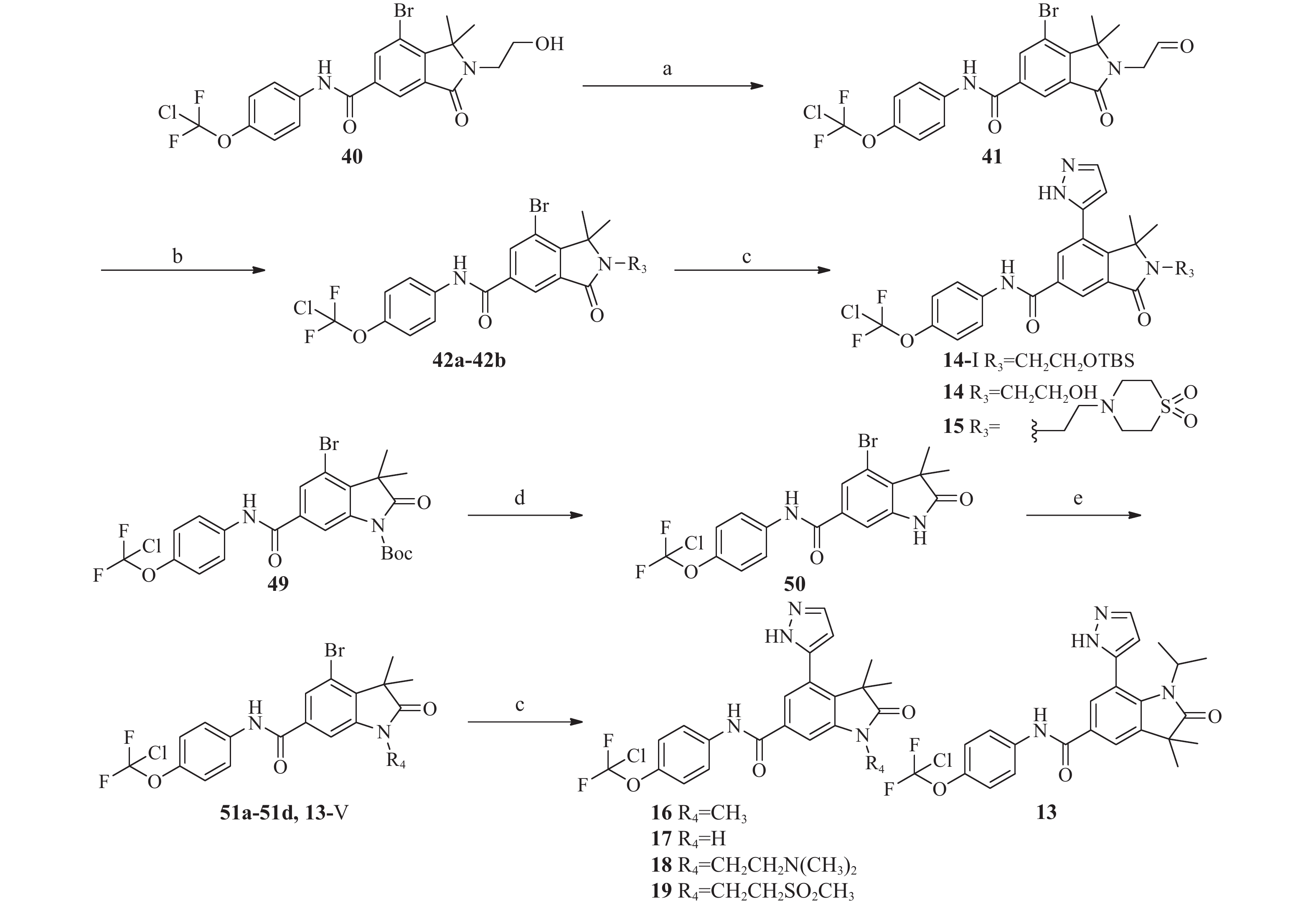
化合物1~22的合成见图4~5,相关中间体的合成路线以及相关化合物表征数据见补充材料。
![]() Figure 4. Synthetic route of compound 1–12,20–22Reagents and conditions: (a) LiOH, THF; (b)4-chlorodifluoromethoxyaniline, HATU, TEA, DMF; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(Ⅱ)[Pd(dppf)Cl2](Ⅱ), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h
Figure 4. Synthetic route of compound 1–12,20–22Reagents and conditions: (a) LiOH, THF; (b)4-chlorodifluoromethoxyaniline, HATU, TEA, DMF; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(Ⅱ)[Pd(dppf)Cl2](Ⅱ), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h![]() Figure 5. Synthetic route of compound 13-19Reagents and conditions: (a) Oxalyl chloride, DMSO, TEA; (b) R3NH, NaBH(OAc)3, DCM; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′Bis(diphenylphosphino)ferrocene]dichloropalladium(II)[Pd(dppf)Cl2](II), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h; (d) TFA, DCM; (e) NaH, R-Cl, DMF
Figure 5. Synthetic route of compound 13-19Reagents and conditions: (a) Oxalyl chloride, DMSO, TEA; (b) R3NH, NaBH(OAc)3, DCM; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′Bis(diphenylphosphino)ferrocene]dichloropalladium(II)[Pd(dppf)Cl2](II), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h; (d) TFA, DCM; (e) NaH, R-Cl, DMF化合物28a~28h的合成通法 取中间体25a~25d,25h、27e~27g溶于四氢呋喃4 mL中,搅拌溶解后加入1 mol/L氢氧化锂水溶液4 mL,室温反应1 h,TLC(乙酸乙酯-正庚烷,1∶9)确认反应完全后,向反应液中滴加1 mol/L HCl水溶液调节体系pH=4~5,乙酸乙酯萃取(20 mL×3),合并有机相,无水硫酸钠干燥,浓缩,得到中间体28a~28h淡黄色固体,收率90%~98%。
中间体29的合成通法 中间体28a~28h(1 mmol)溶于DMF 10 mL中,搅拌溶解后,加入4-氯二氟甲氧基苯胺(1.1 mmol),三乙胺(1.5 mmol), 2-(7-氮杂苯并三氮唑)-N,N,N′,N′-四甲基脲六氟磷酸酯(HATU,1.1 mmol),45 ℃反应4~5 h,TLC(甲醇-二氯甲烷,1∶9)确认反应完全后,加水10 mL淬灭反应,乙酸乙酯(15 mL×3)萃取,合并有机相,有机相水洗(10 mL×2),饱和食盐水10 mL洗,有机相浓缩,硅胶柱色谱分离(乙酸乙酯-正庚烷,0∶100→25∶75),得到中间体29a~29h淡黄色固体,收率60%~90%。
最终化合物1~7和9的合成通法 中间体29a~29h(1 mmol)溶于1,4-二氧六环12 mL中,搅拌溶解后加入5-(4,4,5,5-四甲基-1,3,2-二氧硼杂环戊烷-2-基)-1-H-吡唑(2 mmol),1,1′-双(二苯膦基)二茂铁二氯化钯(Ⅱ)二氯甲烷复合物(0.2 mmol),饱和碳酸钠水溶液4 mL,氮气保护下,110 ℃反应7 h,加水10 mL淬灭反应,乙酸乙酯(20 mL×3)萃取,合并有机相,有机相水洗(10 mL×2),饱和食盐水10 mL洗,有机相浓缩,硅胶柱色谱分离(乙酸乙酯-正庚烷,15∶85→50∶50),得到白色固体最终化合物1~7和9。
化合物8,10~12和化合物20~22的合成 分别将中间体化合物25a~25d,25h、27e~27g换成中间体31a~31d和52a~52c按照相同合成路径合成。
中间体41的合成 氮气保护下,草酰氯(230 mg,1.8 mmol)溶于二氯甲烷10 mL中,搅拌溶解后,−78 ℃搅拌0.25 h,滴加二甲亚砜(280 mg,3.6 mmol),继续搅拌0.25 h,滴加中间体40(600 mg,1.2 mmol)的二氯甲烷10 mL的溶液,反应1 h后用注射器向反应液中注入三乙胺2 mL,后升温至室温搅拌0.5 h,氯化铵水溶液淬灭,二氯甲烷萃取(30 mL×3),合并有机相,干燥,浓缩,硅胶柱色谱分离(乙酸乙酯-正庚烷, 0∶100→50∶50),得到中间体41淡粉色固体332 mg,收率55.00%。
中间体42的合成
42a 取中间体41(80 mg,0.16 mmol)溶于DMF 5 mL中,搅拌溶解后,加入叔丁基二甲基氯硅烷(36 mg,0.24 mmol),咪唑( 33 mg,0.48 mmol),氮气保护下40 ℃反应2 h,LC-MS确认反应完全后,加水10 mL淬灭反应,乙酸乙酯(15 mL×3)萃取,干燥,浓缩,硅胶柱色谱分离(乙酸乙酯-正庚烷, 0∶100→30∶70),得到中间体42a白色固体60 mg,收率61.00%。
42b 取硫代吗啉-1,1-二氧化物(31 mg,0.21 mmol)溶于二氯甲烷5 mL中,搅拌溶解后,加入中间体41(106 mg,0.23 mmol)、三乙酰氧基硼氢化钠(75 mg,0.63 mmol)以及醋酸(12.6 mg,0.21 mmol),室温反应,薄层色谱法(TLC)检测(乙酸乙酯-正庚烷,1∶4)确认反应完全后,加水10 mL淬灭反应,二氯甲烷(10 mL×3)萃取,合并有机相,浓缩,硅胶柱色谱分离(乙酸乙酯-正庚烷,20∶80→50∶50)得到化合物42b 75 mg,收率57.00%。
中间体50的合成 取中间体49(670 mg,1.2 mmol),溶于二氯甲烷10 mL中,搅拌溶解后加入三氟乙酸2 mL,氮气保护下室温反应3 h,LC-MS确认反应完全后,加水15 mL淬灭反应,二氯甲烷萃取(20 mL×3),合并有机相,有机相用无水硫酸钠干燥,浓缩得到中间体50淡黄色固体545 mg,收率99.00%。
中间体51的合成通法 取氢化钠(NaH,5 mmol)溶于N,N-二甲基甲酰胺(DMF)5 mL中,搅拌溶解后,冰浴下向反应液中滴加中间体50(1 mmol)的DMF 5 mL溶液,滴加完毕后继续冰浴下搅拌0.5 h,后冰浴下向反应液中滴加相应的碘代烷(5 mmol),滴加完毕后将反应液升至室温继续反应3 h,TLC(乙酸乙酯-正庚烷,1∶3)确认反应完成后,向体系中加水10 mL淬灭反应,乙酸乙酯(15 mL×3)萃取,合并有机相,有机相用无水硫酸钠干燥,硅胶柱色谱分离(乙酸乙酯-正庚烷,0∶100→10∶90)得到中间体51a~51d白色固体,收率50%~65%。
最终化合物13~19的合成 按照化合物1~7,9相同方法合成。最终化合物14需在化合物14-Ⅰ的基础上进一步将纯化产物溶于甲醇10 mL中,搅拌溶解后,加入对甲苯磺酸一水合物(0.1 mmol),室温反应0.5 h,LC-MS确认反应完全后,浓缩,经制备液相,得到最终化合物14。最终化合物的表征数据如表1所示,经HPLC检测,所有最终化合物的纯度均大于95%。
Table 1. Characteristics of compound 1-22Compd. Yield/% ESI-MS m/z [M+H]+ 1H NMR (400 MHz, DMSO), δ 13C NMR 1 44.80 447.00 12.93 (1H, s, 2′-NH), 10.08 (1H, s, NH-CO), 7.89 (2H, d, J = 9.1 Hz, Ar-H), 7.69 (3H, s, Ar-H, 4′,5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.41–6.36 (1H, m, Ar-H), 3.47 (2H, t, J = 8.8 Hz, 3-CH2), 3.01 (2H, t, J = 8.8 Hz, 2-CH2), 1.28–1.21 (1H, m, 1′′-CH), 0.89 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 165.60, 152.55, 145.04, 139.46, 132.30, 131.63, 125.50, 122.13, 121.78, 105.48, 46.34, 45.28, 27.52, 19.06 2 50.00 419.07 12.91 (1H, s, 2′-NH), 10.08 (1H, s, NH-CO), 7.88 (2H, d, J = 9.1 Hz, Ar-H), 7.76–7.65 (3H, m, Ar-H, 4′,5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.37 (1H, d, J = 2.0 Hz, Ar-H), 3.45 (2H, t, J = 8.6 Hz, 2-CH2), 3.02 (2H, t, J = 8.6 Hz, 3-CH2), 2.45 (3H, s, NCH3) 165.55, 153.84, 145.05, 139.43, 131.76, 131.45, 125.49, 122.99, 122.64, 122.15, 121.79, 106.27, 56.63, 29.48, 27.82 3 28.60 475.10 12.87 (1H, s, 2′-NH), 10.44 (1H, s, NH-CO), 8.09 (1H, s, 4′-CH), 7.91 (2H, d, J = 9.1 Hz, Ar-H), 7.83 (1H, s, Ar-H), 7.74 (1H, s, 5′-CH), 7.36 (2H, d, J = 9.1 Hz, Ar-H), 6.40 (1H, s, Ar-H), 4.22 (2H, t, J = 7.6 Hz, 2-CH2), 3.11 (2H, t, J = 7.7 Hz, 3-CH2), 2.79 (1H, s, 2′′-CH), 0.89–0.83 (6H, m, 3′′,4′′-CH3) 165.70, 145.41, 143.58, 139.04, 137.07, 131.24, 127.91, 125.48, 122.28, 122.03, 103.65, 32.92, 29.31, 19.36, 14.55 4 41.10 445.00 12.82 (s, 1H, 2′-NH), 10.07 (1H, s, NH-CO), 7.88 (2H, d, J = 9.1 Hz, Ar-H), 7.71–7.69 (1H, m, 4′-CH), 7.67 (1H, d, J = 2.0 Hz, Ar-H), 7.60 (1H, s, 5′-CH), 7.31 (2H, d, J = 8.6 Hz, Ar-H), 6.34 (1H, d, J = 2.0 Hz, Ar-H), 3.53 (2H, t, J = 8.6 Hz, 2-CH2), 2.96 (2H, t, J = 8.5 Hz, 3-CH2), 2.28 (1H, tt, J = 7.0, 3.7 Hz, 1′′-CH), 0.31 (2H, dt, J = 6.8, 3.3 Hz, 2′′-CH2), 0.08 (2H, dt, J = 6.8, 3.3 Hz, 3′′-CH2) 165.42, 153.15, 145.06, 139.41, 132.00, 131.90, 125.49, 122.65, 122.13, 121.82, 106.87, 53.35, 31.61, 27.44, 7.58 5 19.20 459.21 12.94 (1H, s, 2′-NH), 10.06 (1H, s, NH-CO), 7.87 (2H, d, J = 9.1 Hz, Ar-H), 7.79–7.69 (1H, m, 4′-CH), 7.69–7.63 (2H, m, Ar-H, 5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.37 (1H, d, J = 2.0 Hz, Ar-H), 4.03–3.79 (1H, m, 1′′-CH), 3.64 (2H, t, J = 8.8 Hz, 2-CH2), 3.02 (2H, t, J = 8.7 Hz, 3-CH2), 2.14–1.97 (2H, m, 2′′-CH2), 1.71–1.59 (2H, m, 4′′-CH2), 1.31–1.11 (2H, m, 3′′-CH2) 165.51, 151.92, 145.03, 139.43, 131.97, 131.67, 122.14, 121.77, 105.72, 51.51, 47.50, 27.38, 27.13, 14.63 6 23.50 473.00 12.97 (1H, s, 2′-NH), 10.07 (1H, s, NH-CO), 7.88 (2H, d, J = 9.2 Hz, Ar-H), 7.73–7.68 (1H, m, 4′-CH), 7.67 (2H, s, 5′-CH, Ar-H), 7.31 (2H, d, J = 9.2 Hz, Ar-H), 6.38 (1H, d, J = 2.0 Hz, Ar-H), 3.72 (1H, s, 1′′-CH), 3.51 (2H, t, J = 8.8 Hz, 2-CH2), 3.02 (2H, t, J = 8.7 Hz, 3-CH2), 1.48–1.41 (4H, m, 2′′,5′′-CH2), 1.32–1.13 (4H, m, 3′′,4′′-CH2) 165.55, 153.20, 145.01, 139.48, 131.95, 131.79, 122.13, 121.98, 121.77, 105.62, 57.29, 46.53, 27.95, 27.49, 24.06 7 34.30 487.00 12.95 (1H, s, 2′-NH), 10.04 (1H, s, NH-CO), 7.89 (2H, dd, J = 9.1, 2.4 Hz, Ar-H), 7.68 (1H, s, 4′-CH), 7.65 (1H, d, J = 2.3 Hz, Ar-H), 7.64 (1H, s, 5′-CH), 7.30 (2H, dd, J = 9.1, 2.4 Hz, Ar-H), 6.33 (1H, d, J = 2.3 Hz, Ar-H), 3.52–3.42 (2H, m, 2-CH2), 3.40–3.32 (1H, m, 1′′-CH), 3.03–2.94 (2H, m, 3-CH2), 1.62–1.47 (4H, m, 2′′,6′′-CH2), 1.31–1.20 (4H, m, 3′′,5′′-CH2), 0.77–0.67 (2H, m, 4′′-CH2) 165.51, 152.39, 144.99, 139.49, 131.81, 131.64, 125.49, 122.09, 121.74, 105.88, 54.66, 46.71, 29.38, 25.81, 25.53 8 55.10 475.20 12.89 (1H, s, 2′-NH), 10.06 (1H, s, NH-CO), 7.87 (2H, d, J = 9.0 Hz, Ar-H), 7.78 (1H, s, 4′-CH), 7.69 (1H, s, Ar-H), 7.67 (1H, s, 5′-CH), 7.31 (2H, d, J = 8.6 Hz, Ar-H), 6.38 (1H, s, Ar-H), 3.66 (2H, t, J = 9.1 Hz, 2-CH2), 3.01 (1H, dp, J = 8.9, 6.8 Hz, 3-CH), 1.32 (3H, d, J = 6.8 Hz, 3-CCH3), 1.27–1.19 (1H, m, 1′′-CH), 0.93 (3H, d, J = 6.6 Hz, 2′′-CH3), 0.84 (3H, d, J = 6.6 Hz, 3′′-CH3) 151.96, 145.05, 139.43, 137.22, 131.78, 130.44, 128.34, 125.49, 122.33, 122.13, 121.85, 105.53, 53.36, 45.99, 33.87, 20.09, 19.75, 18.54 9 46.60 461.00 12.86 (1H, s, 2′-NH), 10.18 (1H, s, NH-CO), 7.90 (2H, d, J = 9.1 Hz, Ar-H), 7.81 (1H, s, 4′-CH), 7.65 (1H, s, 5′-CH), 7.59 (1H, d, J = 2.3 Hz, Ar-H), 7.32 (2H, d, J = 9.0 Hz, Ar-H), 6.49 (1H, d, J = 2.0 Hz, Ar-H), 3.36 (1H, s, 1′′-CH), 3.16 (2H, t, J = 5.8 Hz, 2-CH2), 2.76 (2H, t, J = 6.2 Hz, 4-CH2), 1.85 (2H, tt, J = 6.1 Hz, 3-CH2), 0.88 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 165.76, 149.65, 145.16, 145.14, 145.12, 139.33, 129.83, 125.49, 124.40, 122.64, 122.17, 121.82, 103.59, 53.07, 41.47, 28.92, 22.73, 19.48 10 78.80 446.00 13.12 (1H, s, 2′-NH), 10.38 (1H, s, NH-CO), 8.36 (1H, s, 4′-CH), 7.97 (2H, d, J = 9.1 Hz, Ar-H), 7.86–7.80 (1H, m, 5′-CH), 7.73 (1H, d, J = 2.1 Hz, Ar-H), 7.67 (1H, d, J = 3.4 Hz, 2-CH), 7.35 (2H, d, J = 9.2 Hz, Ar-H), 6.77 (1H, d, J = 3.3 Hz, 3-CH), 6.54 (1H, d, J = 2.1 Hz, Ar-H), 4.47 (1H, s, 1′′-CH), 1.24 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 166.46, 145.21, 139.36, 135.29, 129.36, 125.51, 125.23, 124.73, 122.19, 121.98, 106.23, 103.71, 47.40, 23.33 11 69.20 459.95 13.08 (1H, s, 2′-NH), 10.37 (1H, s, NH-CO), 8.29 (1H, s, 4′-CH), 7.97 (2H, d, J = 9.1 Hz, Ar-H), 7.91 (1H, s, Ar-H), 7.72 (1H, s, 5′-CH), 7.42 (1H, s, 2-CH), 7.36 (2H, d, J = 9.1 Hz, Ar-H), 6.52 (1H, s, Ar-H), 4.52 (1H, t, J = 7.0 Hz, 1′′-CH), 2.39 (3H, s, 3-CCH3), 1.21 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 166.64, 149.93, 145.24, 139.38, 135.60, 129.52, 125.51, 124.70, 122.18, 122.06, 119.31, 106.05, 47.02, 23.29, 10.07 12 68.00 479.80 13.16 (1H, s, 2′-NH), 10.50 (1H, s, NH-CO), 8.31 (1H, s, 4′- CH), 7.96 (2H, d, J = 9.1 Hz, Ar-H), 7.92 (2H, s, 5′-CH, Ar-H), 7.83–7.78 (1H, m, 2-CH), 7.36 (2H, d, J = 9.0 Hz, Ar-H), 6.57 (1H, d, J = 2.1 Hz, Ar-H), 4.55 (1H, s, 1′′-CH), 1.24 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 165.95, 145.37, 139.15, 134.43, 128.34, 126.26, 126.11, 125.95, 125.49, 122.20, 122.17, 106.22, 105.59, 48.19, 23.15 13 41.40 489.00 13.14 (1H, s, 2′-NH), 10.31 (1H, s, NH-CO), 7.99 (1H, s, 4′- CH), 7.90 (1H, s, Ar-H), 7.90–7.86 (2H, m, Ar-H), 7.83 (1H, s, Ar-H), 7.35 (2H, d, J = 8.5 Hz, Ar-H), 6.52 (1H, s, 5′-CH), 3.74 (1H, s, 1′′-CH), 1.34 (6H, s, 3,3-CCH3), 1.24 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 181.84, 165.28, 145.43, 143.68, 138.95, 136.98, 131.50, 128.01, 125.46, 122.18, 43.23, 24.72 14 53.00 490.92 13.20 (1H, s, 2′-NH), 10.64 (1H, d, J = 2.9 Hz, NH-CO), 8.32 (1H, d, J = 1.7 Hz, Ar-H), 8.24 (1H, d, J = 1.8 Hz, Ar-H), 7.94 (2H, d, J = 9.2 Hz, Ar-H), 7.93–7.89 (1H, m, 4′-CH), 7.38 (2H, d, J = 9.2 Hz, Ar-H), 6.67 (1H, t, J = 2.7 Hz, 5′-CH), 5.76 (1H, d, J = 3.0 Hz, OH), 3.67–3.61 (2H, m, 1′′-CH2), 3.50 (2H, s, 2′′-CH2), 1.57 (6H, d, J = 2.9 Hz, 3,3-CCH3) 165.68, 164.97, 145.33, 138.74, 134.97, 133.27, 132.57, 122.32, 121.84, 106.28, 64.95, 59.17, 42.03, 24.20 15 37.00 608.11 10.65 (1H, d, J = 3.3 Hz, NH-CO), 8.33 (1H, d, J = 1.9 Hz, Ar-H), 8.27 (1H, d, J = 1.9 Hz, Ar-H), 7.97–7.90 (3H, m, Ar-H, 4′-CH), 7.38 (2H, d, J = 8.5 Hz, Ar-H), 6.69 (1H, d, J = 2.4 Hz, 5′-CH), 3.78–3.63 (4H, m, 3′′′,5′′′-CH2), 3.62–3.55 (4H, m, 2′′′,6′′′ -CH2), 3.40–3.36 (2H, m, 1′′-CH2), 3.11–3.02 (2H, m, 2′′-CH2), 1.60 (6H, s, 3,3-CCH3) 174.98, 166.20, 164.64, 138.69, 135.07, 133.44, 130.69, 122.32, 122.07, 106.30, 65.35, 54.13, 51.10, 49.79, 29.47, 24.04 16 35.60 461.00 13.14 (1H, s, 2′-NH), 10.49 (1H, s, NH-CO), 7.93 (2H, d, J = 9.1 Hz, Ar-H), 7.91–7.87 (1H, m, 4′-CH), 7.84 (1H, d, J = 1.6 Hz, Ar-H), 7.55 (1H, d, J = 1.7 Hz, Ar-H), 7.37 (2H, d, J = 8.9 Hz, Ar-H), 6.69 (1H, t, J = 2.2 Hz, 5′-CH), 3.26 (3H,s, NCH3), 1.44 (6H, s, 3,3-CCH3) 181.15, 165.78, 149.35, 145.58, 144.36, 138.82, 135.46, 134.77, 131.57, 129.78, 125.47, 123.52, 122.20, 107.14, 105.69, 45.77, 26.72, 22.83 17 41.00 447.00 13.10 (1H, s, 2′-NH), 10.65 (1H, s, 1-NH), 10.48 (1H, s, NH-CO), 7.91 (2H, d, J = 9.1 Hz, Ar-H), 7.87 (1H, s, 4′-CH), 7.73 (1H, s, Ar-H), 7.39 (1H, s, Ar-H), 7.36 (2H, d, J = 8.7 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 1.41 (6H, s, 3,3-CCH3) 182.97, 170.79, 165.88, 149.45, 145.52, 142.80, 138.86, 136.22, 134.67, 129.74, 125.47, 122.29, 122.14, 108.30, 105.63, 46.08, 22.81 18 36.60 518.00 13.13 (1H, s, 2′-NH), 10.48 (1H, d, J = 12.4 Hz, NH-CO), 7.97–7.86 (3H, m, Ar-H, 4′-CH), 7.79 (1H, s, Ar-H), 7.57 (1H, s, Ar-H), 7.37 (2H, d, J = 8.3 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 3.88 (2H, d, J = 8.3 Hz, 1′′-CH2), 2.57 (2H, d, J = 8.4 Hz, 2′′-CH2), 2.23 (6H, d, J = 5.1 Hz, NCH3), 1.41 (6H, s, 3,3-CCH3) 181.17, 165.77, 145.56, 143.49, 138.79, 134.73, 123.49, 122.23, 107.37, 105.77, 56.04, 45.55, 38.00, 22.94, 22.64 19 59.60 553.18 13.14 (1H, s, 2′-NH), 10.51 (1H, s, NH-CO), 7.92 (2H, d, J = 9.1 Hz, Ar-H), 7.91–7.85 (1H, m, 4′-CH), 7.79 (1H, s, Ar-H), 7.59 (1H, s, Ar-H), 7.40 (2H, d, J = 9.1 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 4.26 (2H, t, J = 6.7 Hz, 1′′-CH2), 3.60 (2H, t, J = 6.6 Hz, 2′′-CH2), 3.12 (3H, s, SCH3), 1.43 (6H, s, 3,3-CCH3) 181.21, 167.43, 165.90, 149.29, 145.60, 142.56, 138.79, 134.97, 125.47, 122.35, 122.17, 107.59, 105.73, 50.66, 45.64, 33.75, 22.75 20 47.80 458.90 10.53 (1H, s, 1-NH), 10.46 (1H, s, NH-CO), 7.91 (1H, s, 4′′-CH), 7.89 (2H, d, J = 8.3 Hz, Ar-H), 7.71 (1H, s, Ar-H), 7.37 (2H, d, J = 8.3 Hz, Ar-H), 7.34 (1H, s, 5′′-CH), 6.70 (1H, d, J = 2.3 Hz, Ar-H), 2.85 (2H, dd, J = 20.5, 11.8 Hz, 2′- CH2), 2.24–2.15 (2H, m, 4′-CH2), 1.32–1.21 (2H, m, 3′-CH2) 183.02, 165.64, 145.52, 143.09, 138.83, 134.55, 122.94, 122.28, 122.19, 108.02, 105.75, 48.67, 29.06, 15.59 21 42.70 473.00 13.10–13.05 (1H, m, 2′′-NH), 10.49 (1H, s, 1-NH), 10.44 (1H, s, NH-CO), 7.90 (2H, d, J = 8.3 Hz, Ar-H), 7.86 (1H, s, 4′′-CH), 7.65 (1H, s, Ar-H), 7.36 (1H, s, 5′′-CH), 7.35 (2H, d, J = 8.3 Hz, Ar-H), 6.58 (1H, s, Ar-H), 1.83 (2H, d, J = 17.7 Hz, 2′-CH2), 1.68 (2H, s, 5′-CH2), 1.43–1.28 (2H, m, 4′-CH2), 1.25 (2H, d, J = 4.2 Hz, 3′-CH2) 149.56, 145.50, 143.37, 138.86, 134.33, 132.09, 129.65, 129.15, 125.46, 122.26, 122.12, 105.59, 56.60, 26.91 22 54.80 487.00 13.09 (1H, s, 2′′-NH), 10.47 (1H, s, 1-NH), 10.45 (1H, s, NH-CO), 7.89 (2H, d, J = 9.1 Hz, Ar-H), 7.87 (1H, s, 4′′- CH), 7.59 (1H, d, J = 1.6 Hz, Ar-H), 7.35 (2H, d, J = 9.0 Hz, Ar-H), 7.34 (1H, s, 5′′-CH), 6.57–6.52 (1H, m, Ar-H), 2.09 (2H, tdd, J = 13.2, 10.7, 9.5, 5.7 Hz, 2′-CH2), 1.75–1.53 (2H, m, 6′-CH2), 1.42 (4H, d, J = 12.4 Hz, 3′, 5′-CH2), 1.18 (2H, t, J = 7.1 Hz, 4′-CH2) 182.12, 174.22, 165.83, 145.49, 142.74, 138.87, 134.43, 125.46, 123.64, 122.28, 122.10, 107.96, 106.25, 53.06, 48.07, 24.80, 20.53 1.4 体外活性实验
CCK-8法测定化合物对细胞的毒性实验。将细胞接种到96孔板中,用不同浓度的待测化合物处理72 h(每个化合物选择一系列9个浓度)。将稀释剂100 μL加到同一板中的3~6个孔内,作为细胞对照组,另3~6孔作空白对照组。将细胞混悬液100 μL分配到同一96孔板中,除了空白孔外,然后将96孔板置于37 ℃,5% CO2的培养箱中培养72 h,后每孔加入CCK-8试剂20 μL,继续在37 ℃,5% CO2的培养箱中培养1~4 h。用450 nm微量培养板读数仪检测吸收度A。细胞存活率=(A样品−A空白)/(A细胞对照−A空白)×100%。IC50使用GraphPad Prism 6.0软件计算,结果取两组数据均值。
1.5 肝微粒体稳定性实验
实验分为两组:实验组总孵育体积为250 μL,包括100 mmol/L的磷酸缓冲液216.25 μL,20 mg/mL的小鼠、大鼠或人肝微粒体6.25 μL,10 mmol/L还原型烟酰胺腺嘌呤二核苷酸磷酸(NADPH)25 μL,混合物37 ℃水浴预热10 min,分别加入20 μmol/L阿西米尼和化合物1、13、16、17溶液各2.5 μL,混合液在37 ℃水浴孵育。分别在0.5、5、15、30、60 min时取孵育液30 μL,加入冰乙腈150 μL终止反应,样品在5400 r/min下离心40 min,取上清液100 μL与超纯水100 μL混合后,使用LC-MS进行分析。对照组将NADPH换成100 mmol/L磷酸缓冲液25 μL,其余处理均相同。
使用Excel进行计算,离子色谱图确定峰面积,斜率k由母体药物剩余百分比(实验组与对照组离子峰面积之比)与孵育时间曲线的自然对数线性回归确定。
体外半衰期(t1/2, min):−(0.693/K);体外清除率(CL, μL/min·mg):(0.693/半衰期) ×(孵育体积(μL)/肝微粒体蛋白数量(mg))[12]。
1.6 化合物水溶性测试
按照参考文献[13]的方法,精密称取化合物1和阿西米尼,加水定容至100 mL,分别移取0.5、1、1.5、2、2.5 mL定容至10 mL,用HPLC测定其吸收度,计算线性回归方程。取过量化合物1和阿西米尼,分别加入到一定量的pH1和pH6.5的缓冲液中,37 ℃恒温摇床72 h,离心取上清液,HPLC测其吸收度,根据线性回归方程计算溶解度。
2. 结果与讨论
2.1 先导化合物1的发现
化合物1~12中(表2),化合物1、4、5、6、8、11对野生型融合细胞的抑制活性与阳性对照阿西米尼相当,化合物1、2、10对T315I突变型融合细胞抑制活性显著优于阿西米尼。化合物1对两种融合细胞具有最好的抑制活性。
Table 2. Cell proliferation assay of compound 1-22 and positive control for BCR-ABL1 in BCR-ABL1WT and BCR-ABL1T315I dependent Luc-Ba/F3 cells (IC50)Compd. Luc-Ba/F3
BCR-ABL1WT
IC50/ (μmol/L)Luc-Ba/F3
BCR-ABL1T315I
IC50/ (μmol/L)Compd. Luc-Ba/F3
BCR-ABL1WT
IC50/ (μmol/L)Luc-Ba/F3
BCR-ABL1T315I
IC50/ (μmol/L)1 0.051 0.091 12 0.505 3.274 2 0.177 0.116 13 0.313 1.104 3 1.158 3.841 14 0.121 1.062 4 0.034 0.295 15 0.364 1.750 5 0.041 0.468 16 0.055 0.315 6 0.031 0.272 17 0.046 0.071 7 0.123 0.574 18 0.324 1.535 8 0.020 0.256 19 0.062 0.377 9 0.235 1.054 20 0.174 1.061 10 0.115 0.063 21 0.022 0.136 11 0.050 0.511 22 0.012 0.164 Asciminib 0.028 0.263 进行初步的构效分析,表2中化合物1~7以二氢吲哚为母核,R1为异丙基时化合物的活性最好,改变取代基的大小会导致化合物对T315I突变型融合细胞的抑制活性降低,但是对野生型融合细胞的抑制活性影响不大。当R1为吸电子基时(化合物3),该化合物的取代基团通过共轭作用与母环形成刚性链接,发现该化合物对两种融合细胞几乎无抑制能力,这表明在该位置引入吸电子基以及刚性链接对化合物的活性是不利的。根据分子对接模型(图3-B)以及化合物活性数据显示,该系列化合物在与靶点口袋结合时,化合物的吡唑环发生偏转,与谷氨酸E481残基形成氢键,R1提供一个合适的位阻,维持吡唑环的偏转,因此取代基的大小对化合物活性影响最大。
后续在化合物1的基础上将二氢吲哚母核扩环为四氢喹啉母核,母核增大变相增大了R1,后续合成过程中该化合物的合成难度的增大也证实了这一点,因此该化合物对两种融合细胞抑制活性大幅度地降低。继续在化合物1的基础上在二氢吲哚母核引入双键,试图提高化合物活性,但是结果表明,引入双键对化合物的活性的影响是不利的。后续以化合物1为先导化合物进行进一步评价。
2.2 先导化合物的进一步优化以及候选化合物17的发现
虽然化合物1对两种细胞有较好的抑制活性,但是化合物1在肝微粒体稳定性实验中暴露出清除率高、半衰期短、溶解度差等问题(表3),因此对化合物1进行优化,主要是亲水性改造,通过在化合物1的二氢吲哚母核2位引入羰基将原本的二氢吲哚母核替换为吲哚酮母核(化合物13),化合物13初步解决了清除率高,半衰期短等问题,但是化合物13对两种融合细胞几乎不具备抑制能力,同时因为化合物13的扩展性差,因此后续在化合物13的基础上设计出异吲哚酮类化合物(化合物14、15)和反转的吲哚酮类化合物(化合物16~22),通过在R4引入大极性基团(图2),来提高化合物的溶解度,降低清除率,延长半衰期。
Table 3. Compounds 1,13,16,17 and asciminib liver microsomal stability assayCompd. CL/(μL /min·mg)
(mice/rat/human)t1/2/min
(mice/rat/human)Solubility/(μg/mL)
(pH 1.0/ pH 6.5)1 86.6/236/40.4 16/5.86/34.3 426/0 13 7.6/114/3.2 182/12.2/433 — 16 <2.77/5.8/<2.77 >500/239/>500 — 17 <2.77/5.8/<2.77 >500/365/>500 — Asciminib 6.2/5.2/<2.77 224/267/>500 416/1.22 CL: Clearance; t1/2: Half-life 化合物14相较于化合物13对两种融合细胞的抑制活性有一定的提升,而化合物15相较于化合物13活性下降(表2),当R3为吸电子基时,氢键供体比氢键受体更为有利,小基团比大基团更好。
吲哚酮类化合物13和异吲哚酮类化合物14、15对两种融合细胞几乎无抑制能力,因此,在此基础上进一步改变酰胺键位置,将异吲哚酮母核替换为反转的吲哚酮母核(化合物16~19),发现相较于化合物14,化合物16、17、19对于两种融合细胞的抑制活性都有大幅度的提高,化合物17对于两种融合细胞的抑制活性均强于化合物1,同时化合物17在清除率和半衰期方面数据上与阳性对照阿西米尼相当(表2)。通过对化合物16~19的构效分析发现,R4为H时,化合物具有最好的抑制活性,而当R4存在取代基时,无论取代基的大小均会导致化合物对T315I突变型融合细胞的抑制活性大幅降低,对野生型融合细胞抑制活性影响不大。结合分子对接模拟结果,化合物17的母核的1位N附近没有足够的空间来容纳取代基(图3-C),因此在母核的N上引入取代基会降低化合物与靶点口袋的结合能力,从而导致化合物对两种融合细胞的抑制活性降低。
在化合物17的基础上,保留母核N上无取代基的结构,对吲哚酮3位进行改造,通过螺环来替代原先的二甲基结构。表2结果表明随着螺环的增大,化合物对两种细胞抑制活性提高,其中五元和六元螺环化合物(化合物21和22)对野生型细胞的抑制活性(IC50=0.022/0.012 μmol/L)超过化合物17(IC50=0.046 μmol/L),但是这两个化合物(IC50=0.136/0.164 μmol/L)对T315I突变型细胞抑制活性弱于化合物17(IC50=0.071 μmol/L),同时这一结果与前文中化合物1~7,R1为环烷基时情况相似,在吡唑环侧位引入环烷基会提高化合物对野生型细胞的抑制活性,但同时也会降低化合物对T315I突变型细胞的抑制活性,结合分子对接模拟结果分析,螺环增大会影响吡唑环与谷氨酸E481残基形成氢键,从而影响化合物的活性。
综合以上信息,化合物17在具有超过化合物1的抗细胞增殖活性的同时,在后续评价中,表现出与阳性对照阿西米尼相当的清除率、半衰期数据,该化合物有望于进行更深入的成药性研究以及后临床前研究。
3. 结 论
本研究通过对阿西米尼结构改造获得了N-苯基吲哚啉-5-甲酰胺分子骨架 Ⅰ,在此基础上借助分子对接模拟等方法设计并合成出化合物1~12,体外活性实验发现化合物1、4、6、8对两种BCR-ABL1依赖型Luc-Ba/F3细胞的抑制能力与阳性对照阿西米尼相当,其中化合物1对T3151突变型BCR-ABL1依赖型Luc-Ba/F3细胞的抑制能力超过了阳性对照,但是化合物1在后续评价中暴露出溶解性差、清除率高、半衰期短等问题。后续以化合物1为先导化合物,对化合物1进行亲水性改造,以吲哚酮或异吲哚酮来替换原先的二氢吲哚母核,在此基础上设计出化合物13~22,化合物13~22有效克服了清除率高以及半衰期短的问题,最终筛选出化合物17,化合物17兼具高活性,低清除率,较长半衰期等优点,有望通过后续研究成为新的BCR-ABL1变构抑制剂。
-
Figure 4. Synthetic route of compound 1–12,20–22
Reagents and conditions: (a) LiOH, THF; (b)4-chlorodifluoromethoxyaniline, HATU, TEA, DMF; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(Ⅱ)[Pd(dppf)Cl2](Ⅱ), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h
Figure 5. Synthetic route of compound 13-19
Reagents and conditions: (a) Oxalyl chloride, DMSO, TEA; (b) R3NH, NaBH(OAc)3, DCM; (c) 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole,[1,1′Bis(diphenylphosphino)ferrocene]dichloropalladium(II)[Pd(dppf)Cl2](II), saturated Na2CO3 aqueous solution / dioxane(1/3), 110 ℃, 7 h; (d) TFA, DCM; (e) NaH, R-Cl, DMF
Table 1 Characteristics of compound 1-22
Compd. Yield/% ESI-MS m/z [M+H]+ 1H NMR (400 MHz, DMSO), δ 13C NMR 1 44.80 447.00 12.93 (1H, s, 2′-NH), 10.08 (1H, s, NH-CO), 7.89 (2H, d, J = 9.1 Hz, Ar-H), 7.69 (3H, s, Ar-H, 4′,5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.41–6.36 (1H, m, Ar-H), 3.47 (2H, t, J = 8.8 Hz, 3-CH2), 3.01 (2H, t, J = 8.8 Hz, 2-CH2), 1.28–1.21 (1H, m, 1′′-CH), 0.89 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 165.60, 152.55, 145.04, 139.46, 132.30, 131.63, 125.50, 122.13, 121.78, 105.48, 46.34, 45.28, 27.52, 19.06 2 50.00 419.07 12.91 (1H, s, 2′-NH), 10.08 (1H, s, NH-CO), 7.88 (2H, d, J = 9.1 Hz, Ar-H), 7.76–7.65 (3H, m, Ar-H, 4′,5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.37 (1H, d, J = 2.0 Hz, Ar-H), 3.45 (2H, t, J = 8.6 Hz, 2-CH2), 3.02 (2H, t, J = 8.6 Hz, 3-CH2), 2.45 (3H, s, NCH3) 165.55, 153.84, 145.05, 139.43, 131.76, 131.45, 125.49, 122.99, 122.64, 122.15, 121.79, 106.27, 56.63, 29.48, 27.82 3 28.60 475.10 12.87 (1H, s, 2′-NH), 10.44 (1H, s, NH-CO), 8.09 (1H, s, 4′-CH), 7.91 (2H, d, J = 9.1 Hz, Ar-H), 7.83 (1H, s, Ar-H), 7.74 (1H, s, 5′-CH), 7.36 (2H, d, J = 9.1 Hz, Ar-H), 6.40 (1H, s, Ar-H), 4.22 (2H, t, J = 7.6 Hz, 2-CH2), 3.11 (2H, t, J = 7.7 Hz, 3-CH2), 2.79 (1H, s, 2′′-CH), 0.89–0.83 (6H, m, 3′′,4′′-CH3) 165.70, 145.41, 143.58, 139.04, 137.07, 131.24, 127.91, 125.48, 122.28, 122.03, 103.65, 32.92, 29.31, 19.36, 14.55 4 41.10 445.00 12.82 (s, 1H, 2′-NH), 10.07 (1H, s, NH-CO), 7.88 (2H, d, J = 9.1 Hz, Ar-H), 7.71–7.69 (1H, m, 4′-CH), 7.67 (1H, d, J = 2.0 Hz, Ar-H), 7.60 (1H, s, 5′-CH), 7.31 (2H, d, J = 8.6 Hz, Ar-H), 6.34 (1H, d, J = 2.0 Hz, Ar-H), 3.53 (2H, t, J = 8.6 Hz, 2-CH2), 2.96 (2H, t, J = 8.5 Hz, 3-CH2), 2.28 (1H, tt, J = 7.0, 3.7 Hz, 1′′-CH), 0.31 (2H, dt, J = 6.8, 3.3 Hz, 2′′-CH2), 0.08 (2H, dt, J = 6.8, 3.3 Hz, 3′′-CH2) 165.42, 153.15, 145.06, 139.41, 132.00, 131.90, 125.49, 122.65, 122.13, 121.82, 106.87, 53.35, 31.61, 27.44, 7.58 5 19.20 459.21 12.94 (1H, s, 2′-NH), 10.06 (1H, s, NH-CO), 7.87 (2H, d, J = 9.1 Hz, Ar-H), 7.79–7.69 (1H, m, 4′-CH), 7.69–7.63 (2H, m, Ar-H, 5′-CH), 7.31 (2H, d, J = 9.1 Hz, Ar-H), 6.37 (1H, d, J = 2.0 Hz, Ar-H), 4.03–3.79 (1H, m, 1′′-CH), 3.64 (2H, t, J = 8.8 Hz, 2-CH2), 3.02 (2H, t, J = 8.7 Hz, 3-CH2), 2.14–1.97 (2H, m, 2′′-CH2), 1.71–1.59 (2H, m, 4′′-CH2), 1.31–1.11 (2H, m, 3′′-CH2) 165.51, 151.92, 145.03, 139.43, 131.97, 131.67, 122.14, 121.77, 105.72, 51.51, 47.50, 27.38, 27.13, 14.63 6 23.50 473.00 12.97 (1H, s, 2′-NH), 10.07 (1H, s, NH-CO), 7.88 (2H, d, J = 9.2 Hz, Ar-H), 7.73–7.68 (1H, m, 4′-CH), 7.67 (2H, s, 5′-CH, Ar-H), 7.31 (2H, d, J = 9.2 Hz, Ar-H), 6.38 (1H, d, J = 2.0 Hz, Ar-H), 3.72 (1H, s, 1′′-CH), 3.51 (2H, t, J = 8.8 Hz, 2-CH2), 3.02 (2H, t, J = 8.7 Hz, 3-CH2), 1.48–1.41 (4H, m, 2′′,5′′-CH2), 1.32–1.13 (4H, m, 3′′,4′′-CH2) 165.55, 153.20, 145.01, 139.48, 131.95, 131.79, 122.13, 121.98, 121.77, 105.62, 57.29, 46.53, 27.95, 27.49, 24.06 7 34.30 487.00 12.95 (1H, s, 2′-NH), 10.04 (1H, s, NH-CO), 7.89 (2H, dd, J = 9.1, 2.4 Hz, Ar-H), 7.68 (1H, s, 4′-CH), 7.65 (1H, d, J = 2.3 Hz, Ar-H), 7.64 (1H, s, 5′-CH), 7.30 (2H, dd, J = 9.1, 2.4 Hz, Ar-H), 6.33 (1H, d, J = 2.3 Hz, Ar-H), 3.52–3.42 (2H, m, 2-CH2), 3.40–3.32 (1H, m, 1′′-CH), 3.03–2.94 (2H, m, 3-CH2), 1.62–1.47 (4H, m, 2′′,6′′-CH2), 1.31–1.20 (4H, m, 3′′,5′′-CH2), 0.77–0.67 (2H, m, 4′′-CH2) 165.51, 152.39, 144.99, 139.49, 131.81, 131.64, 125.49, 122.09, 121.74, 105.88, 54.66, 46.71, 29.38, 25.81, 25.53 8 55.10 475.20 12.89 (1H, s, 2′-NH), 10.06 (1H, s, NH-CO), 7.87 (2H, d, J = 9.0 Hz, Ar-H), 7.78 (1H, s, 4′-CH), 7.69 (1H, s, Ar-H), 7.67 (1H, s, 5′-CH), 7.31 (2H, d, J = 8.6 Hz, Ar-H), 6.38 (1H, s, Ar-H), 3.66 (2H, t, J = 9.1 Hz, 2-CH2), 3.01 (1H, dp, J = 8.9, 6.8 Hz, 3-CH), 1.32 (3H, d, J = 6.8 Hz, 3-CCH3), 1.27–1.19 (1H, m, 1′′-CH), 0.93 (3H, d, J = 6.6 Hz, 2′′-CH3), 0.84 (3H, d, J = 6.6 Hz, 3′′-CH3) 151.96, 145.05, 139.43, 137.22, 131.78, 130.44, 128.34, 125.49, 122.33, 122.13, 121.85, 105.53, 53.36, 45.99, 33.87, 20.09, 19.75, 18.54 9 46.60 461.00 12.86 (1H, s, 2′-NH), 10.18 (1H, s, NH-CO), 7.90 (2H, d, J = 9.1 Hz, Ar-H), 7.81 (1H, s, 4′-CH), 7.65 (1H, s, 5′-CH), 7.59 (1H, d, J = 2.3 Hz, Ar-H), 7.32 (2H, d, J = 9.0 Hz, Ar-H), 6.49 (1H, d, J = 2.0 Hz, Ar-H), 3.36 (1H, s, 1′′-CH), 3.16 (2H, t, J = 5.8 Hz, 2-CH2), 2.76 (2H, t, J = 6.2 Hz, 4-CH2), 1.85 (2H, tt, J = 6.1 Hz, 3-CH2), 0.88 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 165.76, 149.65, 145.16, 145.14, 145.12, 139.33, 129.83, 125.49, 124.40, 122.64, 122.17, 121.82, 103.59, 53.07, 41.47, 28.92, 22.73, 19.48 10 78.80 446.00 13.12 (1H, s, 2′-NH), 10.38 (1H, s, NH-CO), 8.36 (1H, s, 4′-CH), 7.97 (2H, d, J = 9.1 Hz, Ar-H), 7.86–7.80 (1H, m, 5′-CH), 7.73 (1H, d, J = 2.1 Hz, Ar-H), 7.67 (1H, d, J = 3.4 Hz, 2-CH), 7.35 (2H, d, J = 9.2 Hz, Ar-H), 6.77 (1H, d, J = 3.3 Hz, 3-CH), 6.54 (1H, d, J = 2.1 Hz, Ar-H), 4.47 (1H, s, 1′′-CH), 1.24 (6H, d, J = 6.6 Hz, 2′′,3′′-CH3) 166.46, 145.21, 139.36, 135.29, 129.36, 125.51, 125.23, 124.73, 122.19, 121.98, 106.23, 103.71, 47.40, 23.33 11 69.20 459.95 13.08 (1H, s, 2′-NH), 10.37 (1H, s, NH-CO), 8.29 (1H, s, 4′-CH), 7.97 (2H, d, J = 9.1 Hz, Ar-H), 7.91 (1H, s, Ar-H), 7.72 (1H, s, 5′-CH), 7.42 (1H, s, 2-CH), 7.36 (2H, d, J = 9.1 Hz, Ar-H), 6.52 (1H, s, Ar-H), 4.52 (1H, t, J = 7.0 Hz, 1′′-CH), 2.39 (3H, s, 3-CCH3), 1.21 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 166.64, 149.93, 145.24, 139.38, 135.60, 129.52, 125.51, 124.70, 122.18, 122.06, 119.31, 106.05, 47.02, 23.29, 10.07 12 68.00 479.80 13.16 (1H, s, 2′-NH), 10.50 (1H, s, NH-CO), 8.31 (1H, s, 4′- CH), 7.96 (2H, d, J = 9.1 Hz, Ar-H), 7.92 (2H, s, 5′-CH, Ar-H), 7.83–7.78 (1H, m, 2-CH), 7.36 (2H, d, J = 9.0 Hz, Ar-H), 6.57 (1H, d, J = 2.1 Hz, Ar-H), 4.55 (1H, s, 1′′-CH), 1.24 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 165.95, 145.37, 139.15, 134.43, 128.34, 126.26, 126.11, 125.95, 125.49, 122.20, 122.17, 106.22, 105.59, 48.19, 23.15 13 41.40 489.00 13.14 (1H, s, 2′-NH), 10.31 (1H, s, NH-CO), 7.99 (1H, s, 4′- CH), 7.90 (1H, s, Ar-H), 7.90–7.86 (2H, m, Ar-H), 7.83 (1H, s, Ar-H), 7.35 (2H, d, J = 8.5 Hz, Ar-H), 6.52 (1H, s, 5′-CH), 3.74 (1H, s, 1′′-CH), 1.34 (6H, s, 3,3-CCH3), 1.24 (6H, d, J = 6.7 Hz, 2′′,3′′-CH3) 181.84, 165.28, 145.43, 143.68, 138.95, 136.98, 131.50, 128.01, 125.46, 122.18, 43.23, 24.72 14 53.00 490.92 13.20 (1H, s, 2′-NH), 10.64 (1H, d, J = 2.9 Hz, NH-CO), 8.32 (1H, d, J = 1.7 Hz, Ar-H), 8.24 (1H, d, J = 1.8 Hz, Ar-H), 7.94 (2H, d, J = 9.2 Hz, Ar-H), 7.93–7.89 (1H, m, 4′-CH), 7.38 (2H, d, J = 9.2 Hz, Ar-H), 6.67 (1H, t, J = 2.7 Hz, 5′-CH), 5.76 (1H, d, J = 3.0 Hz, OH), 3.67–3.61 (2H, m, 1′′-CH2), 3.50 (2H, s, 2′′-CH2), 1.57 (6H, d, J = 2.9 Hz, 3,3-CCH3) 165.68, 164.97, 145.33, 138.74, 134.97, 133.27, 132.57, 122.32, 121.84, 106.28, 64.95, 59.17, 42.03, 24.20 15 37.00 608.11 10.65 (1H, d, J = 3.3 Hz, NH-CO), 8.33 (1H, d, J = 1.9 Hz, Ar-H), 8.27 (1H, d, J = 1.9 Hz, Ar-H), 7.97–7.90 (3H, m, Ar-H, 4′-CH), 7.38 (2H, d, J = 8.5 Hz, Ar-H), 6.69 (1H, d, J = 2.4 Hz, 5′-CH), 3.78–3.63 (4H, m, 3′′′,5′′′-CH2), 3.62–3.55 (4H, m, 2′′′,6′′′ -CH2), 3.40–3.36 (2H, m, 1′′-CH2), 3.11–3.02 (2H, m, 2′′-CH2), 1.60 (6H, s, 3,3-CCH3) 174.98, 166.20, 164.64, 138.69, 135.07, 133.44, 130.69, 122.32, 122.07, 106.30, 65.35, 54.13, 51.10, 49.79, 29.47, 24.04 16 35.60 461.00 13.14 (1H, s, 2′-NH), 10.49 (1H, s, NH-CO), 7.93 (2H, d, J = 9.1 Hz, Ar-H), 7.91–7.87 (1H, m, 4′-CH), 7.84 (1H, d, J = 1.6 Hz, Ar-H), 7.55 (1H, d, J = 1.7 Hz, Ar-H), 7.37 (2H, d, J = 8.9 Hz, Ar-H), 6.69 (1H, t, J = 2.2 Hz, 5′-CH), 3.26 (3H,s, NCH3), 1.44 (6H, s, 3,3-CCH3) 181.15, 165.78, 149.35, 145.58, 144.36, 138.82, 135.46, 134.77, 131.57, 129.78, 125.47, 123.52, 122.20, 107.14, 105.69, 45.77, 26.72, 22.83 17 41.00 447.00 13.10 (1H, s, 2′-NH), 10.65 (1H, s, 1-NH), 10.48 (1H, s, NH-CO), 7.91 (2H, d, J = 9.1 Hz, Ar-H), 7.87 (1H, s, 4′-CH), 7.73 (1H, s, Ar-H), 7.39 (1H, s, Ar-H), 7.36 (2H, d, J = 8.7 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 1.41 (6H, s, 3,3-CCH3) 182.97, 170.79, 165.88, 149.45, 145.52, 142.80, 138.86, 136.22, 134.67, 129.74, 125.47, 122.29, 122.14, 108.30, 105.63, 46.08, 22.81 18 36.60 518.00 13.13 (1H, s, 2′-NH), 10.48 (1H, d, J = 12.4 Hz, NH-CO), 7.97–7.86 (3H, m, Ar-H, 4′-CH), 7.79 (1H, s, Ar-H), 7.57 (1H, s, Ar-H), 7.37 (2H, d, J = 8.3 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 3.88 (2H, d, J = 8.3 Hz, 1′′-CH2), 2.57 (2H, d, J = 8.4 Hz, 2′′-CH2), 2.23 (6H, d, J = 5.1 Hz, NCH3), 1.41 (6H, s, 3,3-CCH3) 181.17, 165.77, 145.56, 143.49, 138.79, 134.73, 123.49, 122.23, 107.37, 105.77, 56.04, 45.55, 38.00, 22.94, 22.64 19 59.60 553.18 13.14 (1H, s, 2′-NH), 10.51 (1H, s, NH-CO), 7.92 (2H, d, J = 9.1 Hz, Ar-H), 7.91–7.85 (1H, m, 4′-CH), 7.79 (1H, s, Ar-H), 7.59 (1H, s, Ar-H), 7.40 (2H, d, J = 9.1 Hz, Ar-H), 6.67 (1H, s, 5′-CH), 4.26 (2H, t, J = 6.7 Hz, 1′′-CH2), 3.60 (2H, t, J = 6.6 Hz, 2′′-CH2), 3.12 (3H, s, SCH3), 1.43 (6H, s, 3,3-CCH3) 181.21, 167.43, 165.90, 149.29, 145.60, 142.56, 138.79, 134.97, 125.47, 122.35, 122.17, 107.59, 105.73, 50.66, 45.64, 33.75, 22.75 20 47.80 458.90 10.53 (1H, s, 1-NH), 10.46 (1H, s, NH-CO), 7.91 (1H, s, 4′′-CH), 7.89 (2H, d, J = 8.3 Hz, Ar-H), 7.71 (1H, s, Ar-H), 7.37 (2H, d, J = 8.3 Hz, Ar-H), 7.34 (1H, s, 5′′-CH), 6.70 (1H, d, J = 2.3 Hz, Ar-H), 2.85 (2H, dd, J = 20.5, 11.8 Hz, 2′- CH2), 2.24–2.15 (2H, m, 4′-CH2), 1.32–1.21 (2H, m, 3′-CH2) 183.02, 165.64, 145.52, 143.09, 138.83, 134.55, 122.94, 122.28, 122.19, 108.02, 105.75, 48.67, 29.06, 15.59 21 42.70 473.00 13.10–13.05 (1H, m, 2′′-NH), 10.49 (1H, s, 1-NH), 10.44 (1H, s, NH-CO), 7.90 (2H, d, J = 8.3 Hz, Ar-H), 7.86 (1H, s, 4′′-CH), 7.65 (1H, s, Ar-H), 7.36 (1H, s, 5′′-CH), 7.35 (2H, d, J = 8.3 Hz, Ar-H), 6.58 (1H, s, Ar-H), 1.83 (2H, d, J = 17.7 Hz, 2′-CH2), 1.68 (2H, s, 5′-CH2), 1.43–1.28 (2H, m, 4′-CH2), 1.25 (2H, d, J = 4.2 Hz, 3′-CH2) 149.56, 145.50, 143.37, 138.86, 134.33, 132.09, 129.65, 129.15, 125.46, 122.26, 122.12, 105.59, 56.60, 26.91 22 54.80 487.00 13.09 (1H, s, 2′′-NH), 10.47 (1H, s, 1-NH), 10.45 (1H, s, NH-CO), 7.89 (2H, d, J = 9.1 Hz, Ar-H), 7.87 (1H, s, 4′′- CH), 7.59 (1H, d, J = 1.6 Hz, Ar-H), 7.35 (2H, d, J = 9.0 Hz, Ar-H), 7.34 (1H, s, 5′′-CH), 6.57–6.52 (1H, m, Ar-H), 2.09 (2H, tdd, J = 13.2, 10.7, 9.5, 5.7 Hz, 2′-CH2), 1.75–1.53 (2H, m, 6′-CH2), 1.42 (4H, d, J = 12.4 Hz, 3′, 5′-CH2), 1.18 (2H, t, J = 7.1 Hz, 4′-CH2) 182.12, 174.22, 165.83, 145.49, 142.74, 138.87, 134.43, 125.46, 123.64, 122.28, 122.10, 107.96, 106.25, 53.06, 48.07, 24.80, 20.53 Table 2 Cell proliferation assay of compound 1-22 and positive control for BCR-ABL1 in BCR-ABL1WT and BCR-ABL1T315I dependent Luc-Ba/F3 cells (IC50)
Compd. Luc-Ba/F3
BCR-ABL1WT
IC50/ (μmol/L)Luc-Ba/F3
BCR-ABL1T315I
IC50/ (μmol/L)Compd. Luc-Ba/F3
BCR-ABL1WT
IC50/ (μmol/L)Luc-Ba/F3
BCR-ABL1T315I
IC50/ (μmol/L)1 0.051 0.091 12 0.505 3.274 2 0.177 0.116 13 0.313 1.104 3 1.158 3.841 14 0.121 1.062 4 0.034 0.295 15 0.364 1.750 5 0.041 0.468 16 0.055 0.315 6 0.031 0.272 17 0.046 0.071 7 0.123 0.574 18 0.324 1.535 8 0.020 0.256 19 0.062 0.377 9 0.235 1.054 20 0.174 1.061 10 0.115 0.063 21 0.022 0.136 11 0.050 0.511 22 0.012 0.164 Asciminib 0.028 0.263 Table 3 Compounds 1,13,16,17 and asciminib liver microsomal stability assay
Compd. CL/(μL /min·mg)
(mice/rat/human)t1/2/min
(mice/rat/human)Solubility/(μg/mL)
(pH 1.0/ pH 6.5)1 86.6/236/40.4 16/5.86/34.3 426/0 13 7.6/114/3.2 182/12.2/433 — 16 <2.77/5.8/<2.77 >500/239/>500 — 17 <2.77/5.8/<2.77 >500/365/>500 — Asciminib 6.2/5.2/<2.77 224/267/>500 416/1.22 CL: Clearance; t1/2: Half-life -
[1] Burmeister T, Schwartz S, Bartram CR, et al. Patients′ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group[J]. Blood, 2008, 112(3): 918-919.
[2] Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy[J]. J Hematol Oncol, 2018, 11(1): 84.
[3] Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype[J]. Blood, 1996, 88(7): 2375-2384.
[4] Ren RB. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia[J]. Nat Rev Cancer, 2005, 5(3): 172-183.
[5] Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia[J]. Blood, 2009, 113(8): 1619-1630.
[6] Schoepfer J, Jahnke W, Berellini G, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1[J]. J Med Chem, 2018, 61(18): 8120-8135.
[7] Deng XM, Okram B, Ding Q, et al. Expanding the diversity of allosteric bcr-abl inhibitors[J]. J Med Chem, 2010, 53(19): 6934-6946.
[8] Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure[J]. N Engl J Med, 2019, 381(24): 2315-2326.
[9] Cui YW, Zhao RX, Han LL, et al. Advances in the study of new BCR-ABL kinase inhibitors[J]. Acta Pharm Sin (药学学报), 2023, 58(2): 258-273. [10] Robert MC. FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia[EB/OL]. Washington D. C. : Food and Drug Administration, (2021-10-29)[2023-07-24].https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-asciminib-philadelphia-chromosome-positive-chronic-myeloid-leukemia.
[11] Jiang Q, Li ZR, Qin YZ, et al. Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: results of an open-label, multicenter phase 1/2 trial[J]. J Hematol Oncol, 2022, 15(1): 113.
[12] Ruan TT, Ju WJ, Xiong HW, et al. Advances in methodologies for predicting metabolic stability for low-clearance drugs[J]. J China Pharm Univ (中国药科大学学报), 2019, 50(2): 152-160. [13] Huang JK, He LQ, Huang P, et al. Synthesis and biological evaluation of aminoalcohol rheinate as anti-osteosarcoma agents[J]. Acta Pharm Sin (药学学报), 2018, 53(2): 249-255. -
其他相关附件
-
DOCX格式
王永健-补充材料 点击下载(470KB)
-



 下载:
下载: